Test Material Compatibility
The 96-well plate based cytotoxicity assays using NRU are best suited for water-soluble materials; however, test materials may be dissolved in intermediate solvents so that they are compatible with the test system. At IIVS, a solubility test is performed to find the most suitable solvent when the solubility of a test chemical is unknown or unavailable.
As a Prediction of Systemic Toxicity
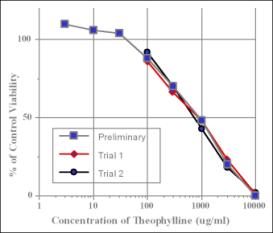
The NHEK Cytotoxicity Assay using a NRU viability endpoint can be used to estimate human lethal serum concentrations, according to a prediction model developed in the Multicentre Evaluation of In Vitro Cytotoxicity (MEIC) program (Part I of this program presents the methodology of 68 in vitro toxicity assays used to test the first 30 chemicals. (MEIC Evaluation of Acute Systemic Toxicity: Part I: C. Clemedson et al, 1996, ATLA 24, 249-272)).
The NHEK Cytotoxicity Assay using a NRU viability endpoint can also be used to estimate starting doses for acute oral toxicity testing in rodents (the methods were initially proposed by ZEBET based upon the correlation of the in vitro cytotoxicity endpoint IC50 and the in vivo LD50 values for 347 chemicals as presented in the Registry of Cytotoxicity (Halle, 2003)).
As a Prediction of Ocular Irritancy
Since the target cells (NHEKs) are epithelial in origin, NHEK Cytotoxicity Assay using a NRU viability endpoint may be used to predict ocular irritation for aqueous soluble materials. This approach has been used to determine the relative ocular irritation potential of surfactants and preservatives, and may be well suited for evaluating relative irritancy potentials of ingredients in ophthalmic solutions in a product development setting.
Specialized Protocols
Test article concentrations may be adjusted as necessary to accommodate specific physical characteristics or client needs. Specialized protocols may be prepared as requested through consultation with the Study Director.
Validation
In 2007, ICCVAM recommended both in vitro test methods (3T3 NRU and NHEK NRU) as reduction alternatives to estimate the starting dose in the Up-and-Down Procedure and Fixed Dose Procedure for assessing acute oral systemic toxicity. Recommendations were accepted by U.S. Federal agencies, and OECD Guidance Document 129 for implementation of the test methods was published in 2010.