Skin Irritation Test (SIT) in a Reconstructed Human Epidermis (RhE) Model
Skin Irritation in the regulatory hazard classification and labeling context is defined as the production of reversible damage to skin following a defined chemical exposure. The Skin Irritation Test (SIT) is an in vitro, non-animal test designed to identify those chemicals and mixtures capable of inducing moderate skin irritation (UN GHS Category 2 Skin Irritants1), and to discriminate UN GHS Category 2 Skin Irritants from UN GHS 3 Mild Skin Irritants as well as those not requiring classification for skin irritation potential.
This test method is useful for regulatory classification and labeling of chemicals and mixtures, and depending upon the regulatory jurisdiction and framework the test results may be used as standalone or to support a weight of evidence approach. Since skin irritation in vivo typically results from chemical-induced cell damage and subsequent inflammatory cascade, this test method provides a mechanistically-relevant measurement of cell viability in reconstructed human epidermis (RhE) tissues after a single topically-applied chemical treatment, relative to negative control-treated tissues. Chemicals which reduce relative viability to ≤ 50% would be classified as an irritant (and a potential corrosive), while those which result in a relative viability > 50% would not be classified as a skin irritant in accordance with UN GHS Category 2.
IIVS has extensive expertise with a wide variety of reconstructed skin-based protocols and participated in the pre-validation and validation studies that led to the adoption of the OECD Test Guideline 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method (TG 439).
NOTE: Since this test method does not discriminate UN GHS categories 1 and 2, an irritant prediction by this test method should be followed up with a test for potential skin corrosion, using any of the validated corrosivity test methods. 1) Corrositex Membrane Barrier Time Monitor Corrosion Test Method and 2) In Vitro Skin Corrosion Test using a Reconstructed Human Epidermis (RhE) Model
A variety of other protocols are available to evaluate for potential skin irritation to support product development, product stewardship, candidate formulation selection and other non-regulatory applications, and can provide a rank ordering of skin irritation potential. A variety of protocols allow for evaluation of mild formulations: 1) Time-to-Toxicity ET50 Screening protocol, and 2) Cytokine Expression Assay for Mild Products and for moderate to corrosive products and mixtures: 3) In Vitro Skin Irritation / Corrosion Screen
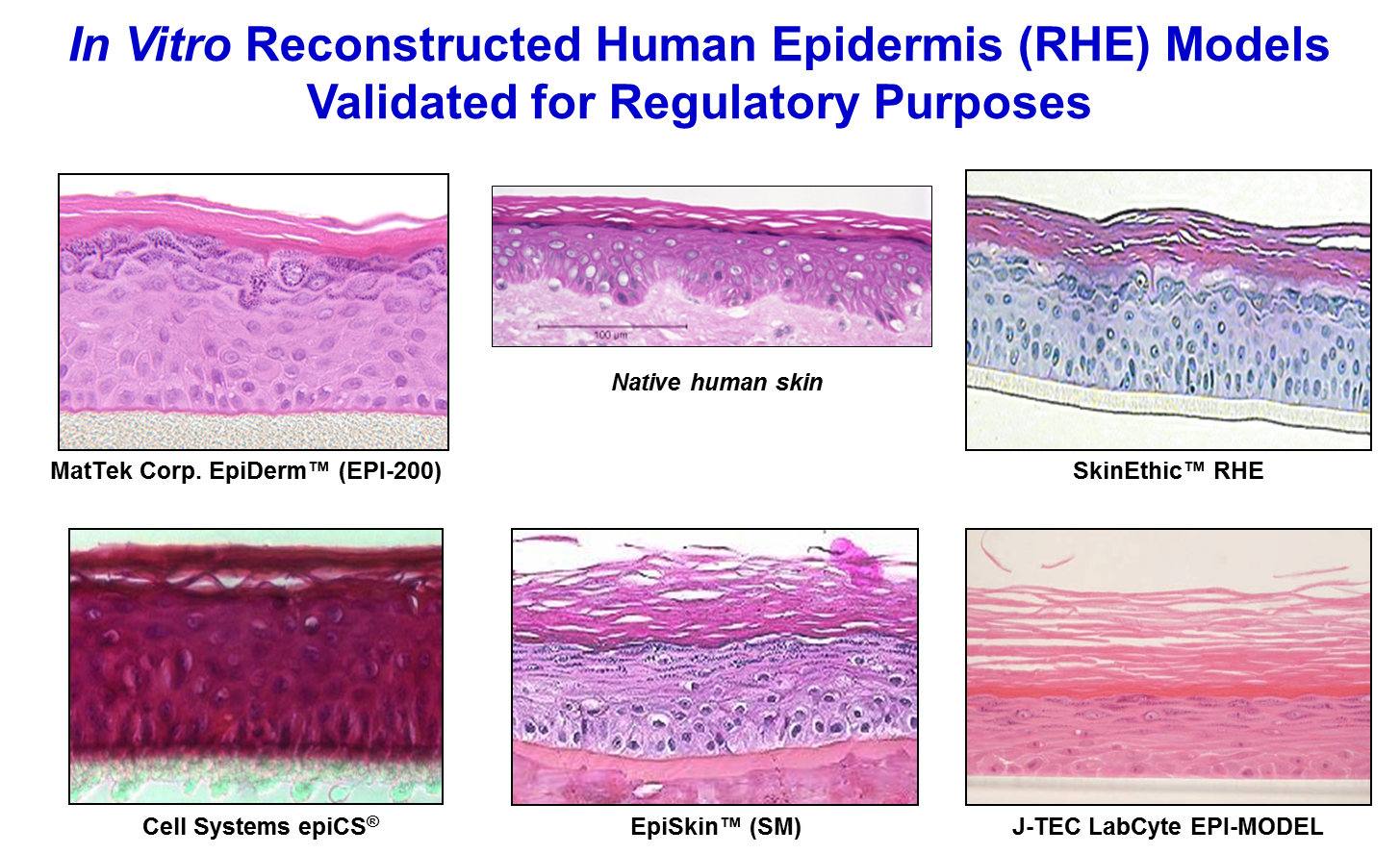
3-D reconstructed human epidermis (RhE) models such as the EpiDerm™ (MatTek Corp.), epiCS® (CellSystems), LabCyte EPI-MODEL (Japan Tissue Engineering Co., Ltd. ), and EpiSkin™ and SkinEthic™ RHE (EpiSkin SA) are organotypic in vitro models of human epidermis which can be utilized in a variety of assays to evaluate the dermal irritation, corrosivity, cytotoxicity, phototoxicity, and/or anti inflammatory potential of test materials. Viability of the tissues is determined using the vital dye MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide). The reduction of MTT in test material-treated tissues is expressed as a percentage relative to negative control-treated cultures.
The EpiDerm™, epiCS®, LabCyte EPI-MODEL, EpiSkin™ and SkinEthic™ RHE tissue models are made from human epithelial cells, which are cultured on specially designed cell culture inserts. The cells differentiate to form a fully differentiated epidermis, complete with a functional stratum corneum (see picture below). An advantage of using 3-D RhE tissues is that test materials are applied topically, at full formulation strength, without dilution, so that most forms of test materials can be applied to the cultures in the same manner as occurs in vivo.