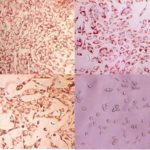
The 3T3 Neutral Red Uptake (NRU) Cytotoxicity Assay using a Neutral Red Uptake (NRU) viability endpoint is a 96-well cytotoxicity assay to assess the toxicity potential of a test material. The assay utilizes Balb/c 3T3 mouse fibroblasts to assess the systemic toxicity potential of a test material and may be used to predict in vivo rodent LD50 starting doses for acute oral systemic toxicity. Cytotoxicity caused by the test article is measured by a concentration-dependent reduction in neutral red dye uptake by the cells after exposure to a test material.
Healthy mammalian cells, when maintained in culture, continuously proliferate. A toxic chemical, regardless of site or mechanism of action, will interfere with this process and result in a reduction in viable cells relative to untreated controls. The viability of the cells can be assessed using a neutral red uptake endpoint. A decrease in the uptake of neutral red dye in treated cell cultures following a test chemical exposure is used to determine relative toxicity.