The majority of the products manufactured or sold by the Household and Cleaning Products industry are subject to the requirements of the Federal Hazardous Substances Act (FHSA) which mandates that the product have appropriate safety labeling. The FHSA does not specifically require animal testing be conducted to determine if a hazard is present, but historically many companies have used animal testing for this purpose – and have continued to do so because of lack of familiarity with newer, non-animal methods.
IIVS is able to be a significant resource for this industry because our 20 year history of conducting in vitro (non-animal) testing in our laboratories gives us the experience to know when and where to use in vitro assays. We can assist you in determining what tests are acceptable to the Consumer Product Safety Commission (which administrates the FHSA) and how to best design your studies.
In a few cases household products may be regulated by another federal agency, such as the Environmental Protection Agency (EPA) or the Food and Drug Agency. Two examples are cleaning products making an antimicrobial claim, and insect repellants, which are regulated by EPA. Again IIVS is able to recommend and conduct non-animal tests which meet the requirements of the agency. In fact, IIVS has worked with the EPA and anti-microbial cleaning product manufacturers to develop a specific non-animal testing strategy to determine the eye irritation potential of pesticide products.
Use of an Alternate Testing Framework for Classification of Eye Irritation Potential of EPA Pesticide Products
A collaboration led by IIVS, in conjunction with the Accord Group, seven manufacturers of anti-microbial cleaning products, and the US EPA resulted in the development of a decision tree approach (See figure 1) using three in vitro or ex vivo assays under the United States Environmental Protection Agency (U.S. EPA) Office of Pesticide Programs (OPP). The three assays are:
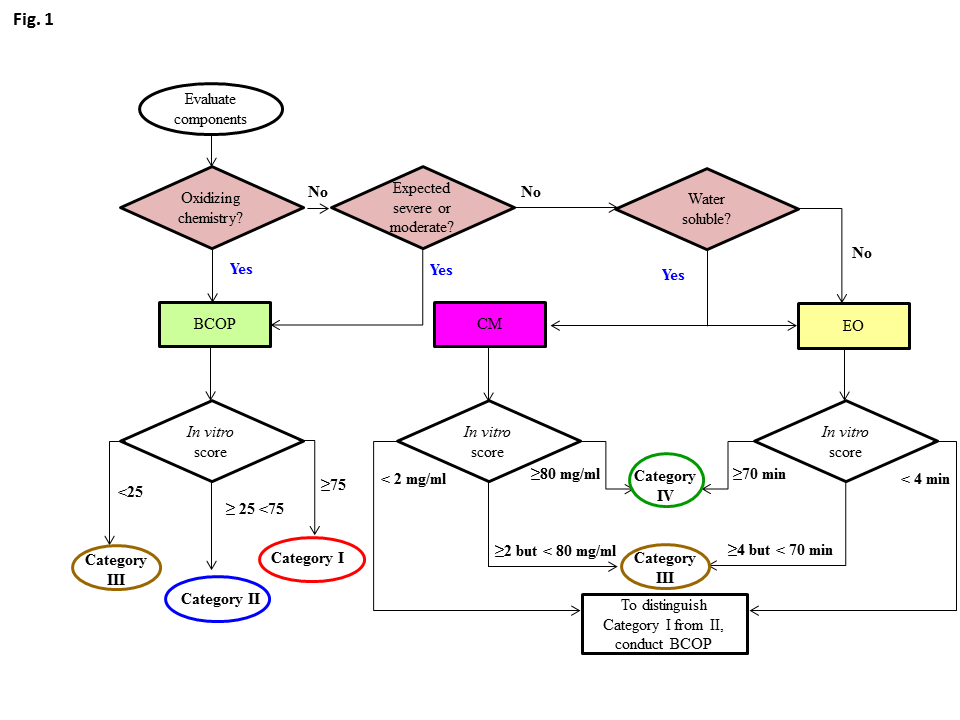
Fig. 1: Decision tree: selection and evaluation of in vitro assays for hazard labeling when using an alternate testing framework for classification of eye irritation potential of US EPA pesticide products. Abbreviations: BCOP = Bovine Corneal Opacity
Data analyses show that these assays can be used either singly or in combination to appropriately label anti-microbial cleaning products (AMCPs) with one of the four US EPA OPP eye irritation hazard categories. Key Points
- For other classes of pesticides and pesticide products, including conventional, biochemical, and other antimicrobial pesticides not in the scope of those with cleaning claims, the US EPA will consider alternative tests conducted and submitted on a case-by-case basis. An IIVS scientist can discuss approaches with you.
- Assay can be used in a top-down approach for determination of EPA Toxicity Categories I, II, and III for eye irritation while the CM and EO assays can be used in a bottom-up approach for determining Toxicity Category IV, III, or I AMCPs.
- The first step in determining the appropriate assay is whether the test material has oxidizing chemistry (e.g., hypochlorite, peroxide, percarbonate, oxygen bleaches, strong acids, etc.) or may be expected to be a severe or moderate eye irritant. If yes to either of these questions, the BCOP assay should be used.
- If the test material does not possess oxidizing chemistry or is not expected to be a severe or moderate eye irritant, the CM or EO assays can be conducted.
- These assays are sensitive to small amounts of damage, and thus are well suited to resolve away the mildest AMCP formulations.
The above guidance provided in: Use of an Alternate Testing Framework for Classification of Eye Irritation Potential of EPA Pesticide Products, US Environmental Protection Agency, Office of Pesticide Programs, Washington, DC, 3/2/2015.
