Product development/product stewardship scientists and formulators typically find it desirable to compare or rank order the skin irritation potential of candidate formulations, or to monitor the impact of changes in formulations or ingredients on skin tolerance. Foremost, industry researchers need timely, accurate, cost-effective test results to help them get safer products to the marketplace.
The Dermal Irritation Screening assay using a Time-to-Toxicity (ET50) protocol coupled with IIVS’ expertise in the methods help researchers meet their testing goals. The assay is designed to determine the test material exposure time that induces a 50% reduction in tissue viability (ET50 time) in a reconstructed human epidermis (RhE) model, relative to negative or solvent controls. This approach allows for a wide range of responses across the corrosion/irritation continuum notably outside the limited scope of regulatory classification and labeling tests. Rather than classify a test material’s irritation potential into discrete regulatory categories, the ET50 screening protocol allows one to determine the irritation potential for materials covering a wide range of irritation responses from highly irritant materials to those which are extremely well tolerated.
NOTE: Since the Dermal Irritation Screening assay using a Time-to-Toxicity (ET50) protocol is not designed for regulatory classification and labeling, the following validated test methods may be used to classify and label the skin irritation or corrosion hazards of test materials according to the United Nations (UN) Globally Harmonized System (GHS) of Classification and Labelling of Chemicals:
- OECD Test Guideline 431: In Vitro Skin Corrosion: Reconstructed Human Epidermis Test Method (TG 431)
- OECD Test Guideline 435: In Vitro Membrane Barrier Test Method for Skin Corrosion (TG 435)
- OECD Test Guideline 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method (TG 439).
IIVS has extensive expertise with a wide variety of reconstructed skin-based protocols and participated in various validation studies that led to the adoption of the test methods listed above.
A variety of other protocols are available to screen for potential skin irritation or corrosion, or local skin tolerance to support product development and product stewardship, and other non-regulatory applications:
-
-
- Cytokine Expression Assay for Mild Products
- In Vitro Skin Irritation / Corrosion Screen for moderate to corrosive products and mixtures
-
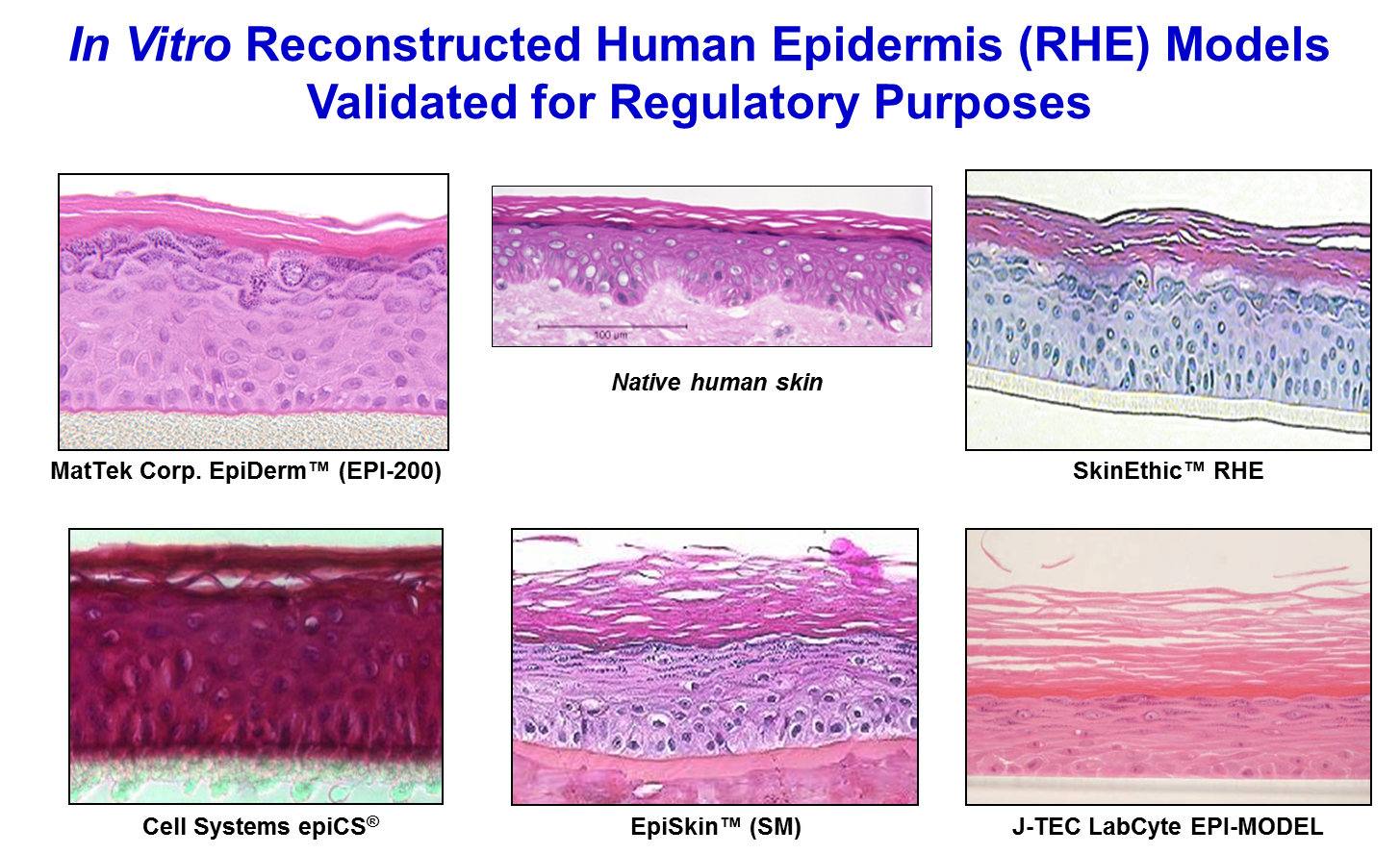
3-D reconstructed human epidermis (RhE) models such as the EpiDerm™ (MatTek Corp.), epiCS® (CellSystems), LabCyte EPI-MODEL (Japan Tissue Engineering Co., Ltd. ), and EpiSkin™ and SkinEthic™ RHE (EpiSkin SA) are organotypic in vitro models of human epidermis which can be utilized in a variety of assays to evaluate the dermal irritation, corrosivity, cytotoxicity, phototoxicity, and/or anti inflammatory potential of test materials. Viability of the tissues is determined using the vital dye MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide). The reduction of MTT in test material-treated tissues is expressed as a percentage relative to negative control-treated cultures.
The EpiDerm™, epiCS®, LabCyte EPI-MODEL, EpiSkin™ and SkinEthic™ RHE tissue models are made from human epithelial cells, which are cultured on specially designed cell culture inserts. The cells differentiate to form a fully differentiated epidermis, complete with a functional stratum corneum (see picture below). An advantage of using 3-D RhE tissues is that test materials are applied topically, at full formulation strength, without dilution, so that most forms of test materials can be applied to the cultures in the same manner as occurs in vivo.