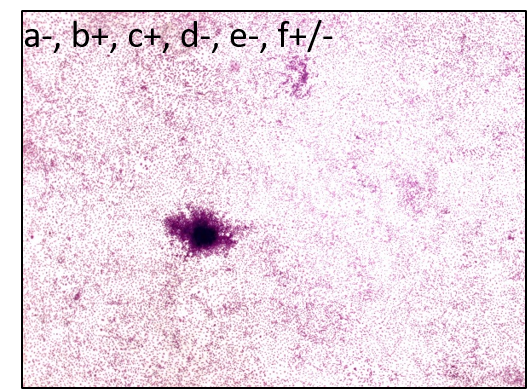
Bhas 42 Cell Transformation Assay (Bhas 42 CTA)
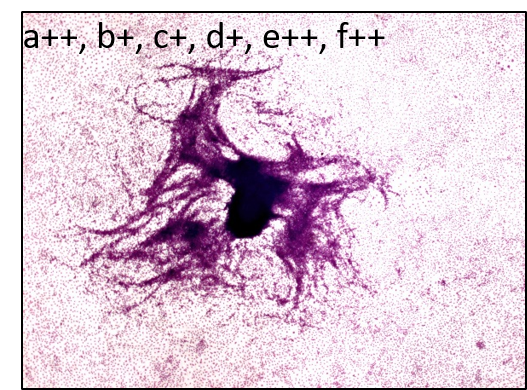
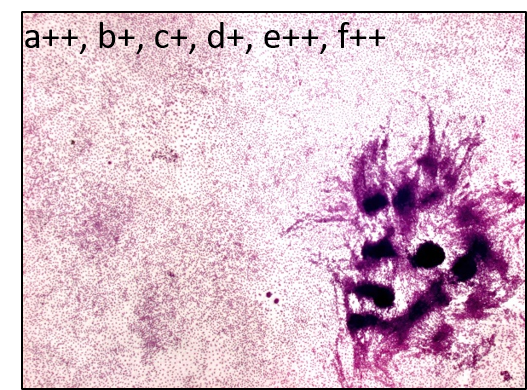
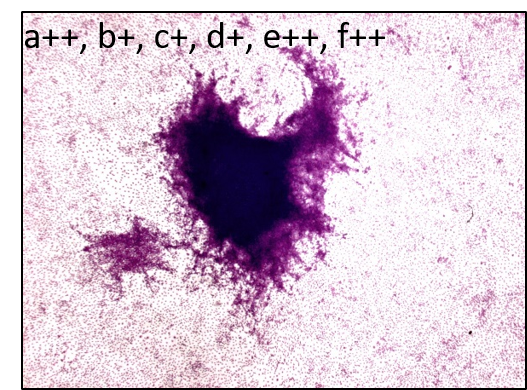
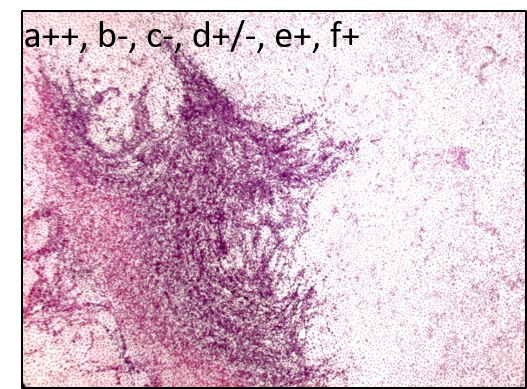
The Bhas 42 CTA is used to evaluate the carcinogenic potential (tumor-initiating activity and/or -promoting activity) of genotoxic or non-genotoxic test articles. The untransformed Bhas 42 cells grow to confluence forming a density-dependent contact-inhibited monolayer. A carcinogenic stimulus induces morphological alterations and leads to the formation of discrete anchorage-independent altered colonies referred to as transformed foci, atop the confluent monolayer. This measure of transformation frequency, which can be extrapolated to an assessment of carcinogenic potential, constitutes the basis of the Bhas 42 CTA.
The Bhas 42 CTA is performed based on the test method provided in OECD’s “Guidance Document on the In Vitro Bhas 42 Cell Transformation Assay; Series on Testing and Assessment No. 231”.
The assay includes two tests, the initiation test to detect potential tumor-initiating activity and the promotion test to determine the tumor-promoting activity of chemicals, but each test can be performed independently. Each test consists of two phases – initial cell growth assay (phase A1) to determine the appropriate test article dose range and cell transformation assay (phase B1).
Test results derived from the Bhas 42 CTA may be used as part of a testing strategy and/or in a weight-of-evidence approach to predicting carcinogenic potential.