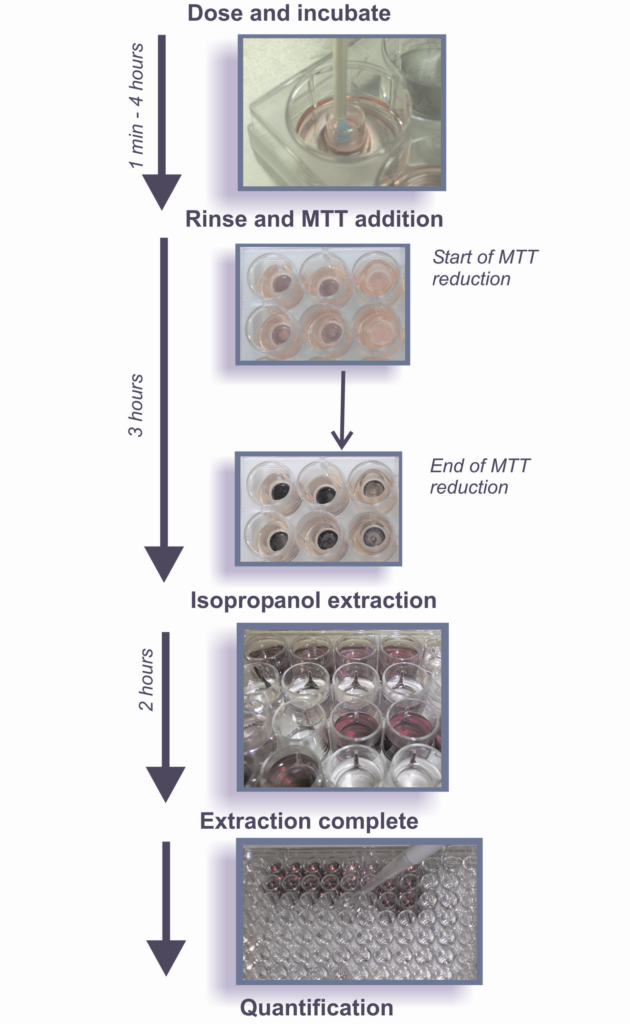
The irritation potential of pharmaceutical, cosmetic and personal care products, medical devices and related consumables (such as lubricants)* that are formulated for application onto human cervical or vaginal tissues can be evaluated using reconstructed organotypic models representative of human ecto-cervical and vaginal tissue. These models overcome some of the disadvantages of monolayer culture by the presence of a barrier layer that permits the direct apical application of active ingredients and final formulations, including mixture, formulations and ingredients that are not water-soluble. Moreover, the structure and morphology of these models is more representative of human tissue than the in vivo (typically rabbit-based) model. The toxicity is determined by measuring the relative viability of the tissues treated with the test materials and compared to the negative/solvent control-treated tissues.
The use of these models avoids animal welfare and inter-species extrapolation issues, and they allow a separation between the very mild products to which animal models are insensitive. The toxicity of the test materials is generally determined by measuring the relative conversion of MTT (3-[4,5 – dimethylthiazol-2-yl] – 2,5 – diphenyltetrazolium bromide) in the test material-treated tissues compared to the negative/solvent control-treated tissues and is evaluated on the basis of the relative tissue viability versus exposure time. The toxicity is expressed as ET50 values representing the exposure time to a test article that reduces the viability of the tissues by 50% and they are dependent on the class of materials tested. Alternative assays that can be used to assess the toxicity of test materials expressed as tissue viability are LDH (lactate dehydrogenase) or ATP (adenosine triphosphate). In addition to tissue viability, other endpoints that can be used to compile the safety profile of test materials are: cytokine release, TEER (transepithelial electrical resistance) and histopathology.
*Although not currently accepted by FDA, IIVS has created an Industry Consortium comprised of manufacturers of personal lubricants/vaginal moisturizers and companies interested in the advancement of animal alternatives working collaboratively with stakeholders and the US FDA to develop an in vitro testing approach that could be used in place of the rabbit vaginal irritation (RVI) in pre-market submissions. View the poster for further information.