Determination of skin sensitization potential is a critical toxicological endpoint in the safety assessment of new chemicals. Although the Guinea Pig Maximization Test (GPMT) and in vivo Local Lymph Node assay (LLNA) have traditionally been used to assess skin sensitization, recent activity has focused on the development of novel non-animal assays for this endpoint. As a culmination of these efforts, the Organization for Economic Cooperation and Development (OECD) has published test guidelines for non-animal skin sensitization testing to address several Key Events in the Adverse Outcome Pathway (indicated in the boxed area below):
Intro to Skin Sensitization
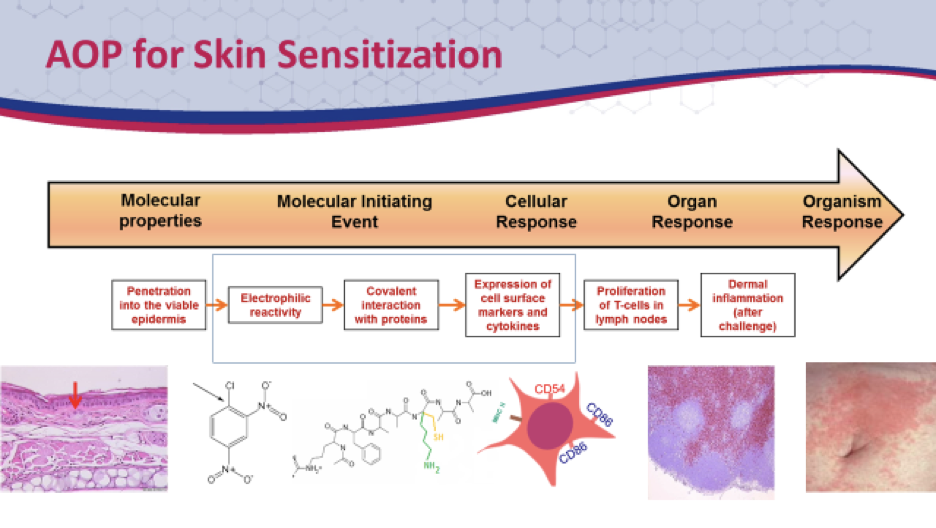
Given the complex cascade of events leading to skin sensitization, it is generally agreed that an integrated testing approach combining multiple assays and in silico predictive tools is needed to fully replace the animal based methods. There is a significant effort underway to ascertain how non-animal assays may be combined to both qualitatively and quantitatively assess skin sensitization most effectively. IIVS is proud to offer a variety of non-animal skin sensitization assays to address several key events of the adverse outcome pathway. Visit our webpage on Integrated Testing Strategies or contact us for information on these skin sensitization assays and how they may be used as part of an integrated testing strategy within your existing testing program.
