The US Environmental Protection Agency (US EPA) continues to drive significant efforts in the United States to modernize the battery of acute toxicity tests classically known as the “6-pack.” Known for many years as an “all in vivo” testing strategy for products registered under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), the 6-pack was revised in 2015 to include non-animal (in vitro/ex vivo) test methods for classification and labeling for Eye Irritation, both for commonly used household cleaning products with anti-microbial claims and more conventional pesticides products.
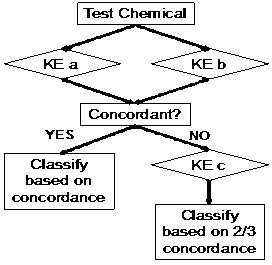
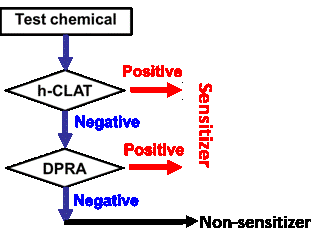
In 2018, EPA has continued the modernization of the 6-pack through its Draft Interim Science Policy to allow the Skin Sensitization endpoint to be performed in in vitro models as well. Currently, only pure substances are permitted to be used as test materials for submission purposes and one of the Integrated Testing Strategies, either the “2 out of 3 Defined Approach” or the “Key Event 3/1 Sequential Testing Strategy” must be followed (see figures 1a and 1b below). It is expected that EPA will release further information regarding the acceptance of mixtures and formulations in the coming months.