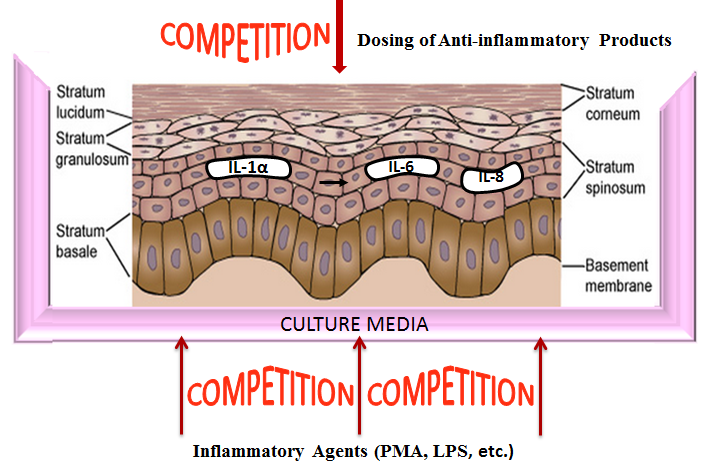
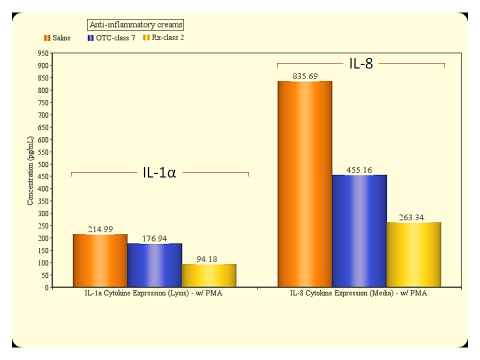
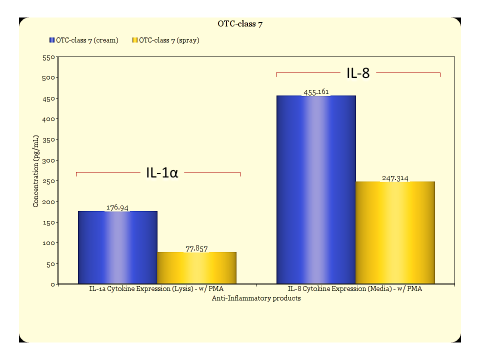
Inflammation is a common effect experienced after encounter with irritating substances, bacterial infection or by those suffering from auto-immune diseases. To address this rather common but impactful condition on quality of life, discovering effective compounds and natural products to counter these effects is a goal for many companies and organizations. Testing with animal models is expensive and time consuming as well as banned in many countries. Fortunately, in vitro models can be an effective tool to accurately evaluate test materials for anti-inflammatory properties.
Reconstructed human epidermal tissues have the capacity to release of (pro)-inflammatory mediators such as interleukin (IL)-1α and IL-8. These cytokines are responsible for the redness and swelling elicited by skin tissues during an inflammatory response. By inducing these cellular products and subsequently treating the tissues with the experimental test article, anti-inflammatory efficacy of the experimental compound or formulation can be determined. A reduction in cytokine response vs. saline control indicates efficacy when the tissues are challenged with an inflammatory agent such as Phorbol 12-myristate 13-acetate (PMA) (see figs. 1 & 2).