The 3-D Phototoxicity assay uses reconstructed human epidermal (RhE) tissue constructs to evaluate the dermal phototoxicity potential of a test material. Toxicity is determined by measuring cytotoxicity [based on the relative conversion of MTT (3-[4,5 – dimethylthiazol-2-yl] – 2,5 – diphenyltetrazolium bromide)] in cultures treated with the test material in the presence and absence of UVA light.
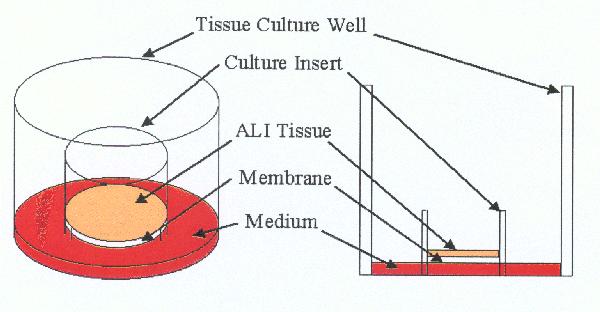
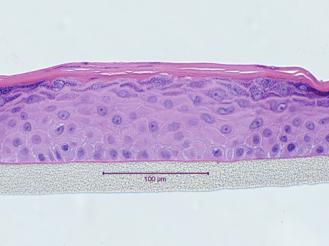
An advantage of using 3-D tissues to evaluate phototoxicity potential is that, unlike the 96-well phototoxicity assay, test materials are applied topically. As such, solids, undiluted final formulations, and insoluble test materials can be tested. RhE tissue constructs are prepared using human epidermal cells cultured on specially designed inserts. The cells differentiate to form a fully differentiated epidermis, complete with a functional stratum corneum (see picture below).
MTT, a vital dye, is actively taken up by the tissues and subsequently reduced in the mitochondria of living cells. This chemical reaction produces a purple-colored formazan within the cells, causing the live tissues to turn deep purple in color. The colored dye can be extracted from the cells and the absorbance read spectrophotometrically.