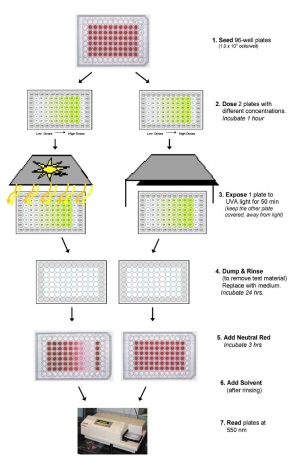
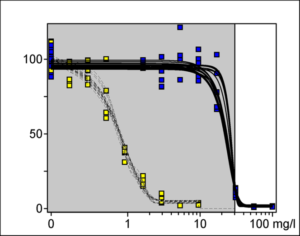
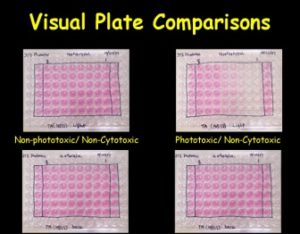
The 3T3 Neutral Red Uptake (NRU) Phototoxicity assay is a 96-well cytotoxicity-based assay that utilizes BALB/c 3T3 mouse fibroblasts to measure the concentration-dependent reduction in neutral red uptake by the cells after exposure to a test material in the presence and absence of UVA light. Duplicate 96-well plates containing a monolayer of 3T3 cells are exposed to serial dilutions of a test material. One of the plates is exposed to 5 J/cm2 of UVA while the other plate is kept in the dark. Neutral red uptake (NRU) by cells exposed to the test chemical in the presence of UVA exposure is compared to the NRU by cells exposed to the test chemical in the absence of UVA exposure to evaluate phototoxicity.
Test Material Compatibility
The assay is well suited for water-soluble materials, and test materials may also be dissolved in intermediate solvents so that they are compatible with the test system. An initial solubility test is performed to find the most suitable solvent when the solubility of a test chemical is unknown.
Test materials that are found to be insoluble or otherwise incompatible with this test system (3T3 NRU Phototox) may be tested using our 3-D Reconstructed Human Epidermis Phototoxicity assay.
Specialized Protocols
The assay and test platform may be adjusted to accommodate specific testing needs. (Read about our work with solid state materials) Specialized protocols may be prepared as requested through consultation with the Study Director.