Determination of skin sensitization potential is a critical endpoint in the safety assessment of raw materials, chemicals, mixtures and formulations. Although the Guinea Pig Maximization Test (GPMT) and Local Lymph node assay (LLNA) have historically been used to address this adverse effect, in vitro assays have been developed and validated in order to replace these resource-intensive animal tests.
Skin sensitization is the result of a series of biochemical “Key Events” (KEs) that involve covalent binding of (generally electrophilic) compounds to cellular proteins (KE1), activation of various pathways within skin cells (KE2) and priming of the immune system (KE3) that results in an allergic response upon repeat exposure to the substance.
IIVS offers multiple assays that evaluate all 3 KEs of skin sensitization initiation, including (and sequentially addressing all 3 KEs) the Direct Peptide Reactivity Assay (DPRA), KeratinoSens™, and the human Cell Line Activation Test (h-CLAT).
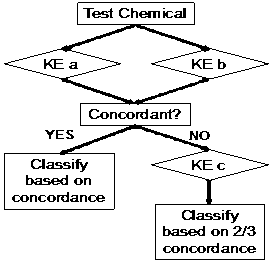
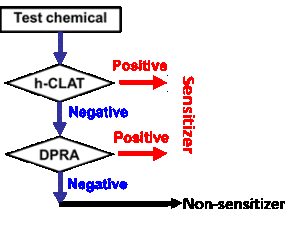
It has been generally accepted that no single assay should be used to confirm sensitization. Proposed Integrated Testing Strategies (ITS) include a “2 out of 3” approach (Figure 1, developed by BASF) and the KE 3/1 sequential approach (Figure 2, developed by Kao). Both of these strategies have been reviewed by OECD and are currently accepted by US EPA as an acceptable in vitro replacement for in vivo methods in the “6-pack” testing requirements for biocide submissions.
Please contact IIVS for more information on individual assays or approaches to in vitro skin sensitization testing that may be suitable for your organization’s testing needs.