Determination of skin sensitization potential is a critical endpoint in the safety assessment of raw materials, chemicals, mixtures and formulations. Although the Guinea Pig Maximization Test (GPMT) and Local Lymph node assay (LLNA) have historically been used to address this adverse effect, in silico and in vitro assays have been developed and validated in order to replace these resource-intensive animal tests.
In 2012, the Organization for Economic Co-operation and Development (OECD) published the Adverse Outcome Pathway (AOP) describing the sequence of molecular and cellular events that lead to the development of allergic contact dermatitis. The first three “Key Events” (KEs) in the AOP which are required for Skin Sensitization are: the covalent binding of compounds to cellular proteins (KE1), activation of various pathways within skin cells (KE2) and priming of the immune system (KE3) that results in an allergic response upon repeat exposure to the substance.
Due largely in part to the complex nature of skin sensitization induction and elicitation, it has been generally accepted that no single assay can be used to fully assess this endpoint.
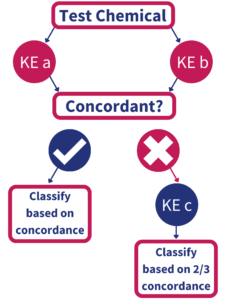
In June 2021, the Organization for Economic Cooperation and Development (OECD) approved the Test Guideline entitled: Defined Approaches to Skin Sensitization, which includes the BASF/Givaudan-developed “2 out of 3” hazard-based approach (Figure 1). According to the Test Guideline, at least 2 of the following three assays which evaluate Key Events 1,2 and 3 must show concordance in order to establish a skin sensitization classification (GHS Category 1 vs. non-classified):
KE1: Direct Peptide Reactivity Assay (DPRA)
KE2: KeratinoSens™
KE3: human Cell Line Activation Test (h-CLAT)
IIVS offers all three tests as well as in silico QSAR analysis based on the OECD Toolbox (OECD TB) platform.
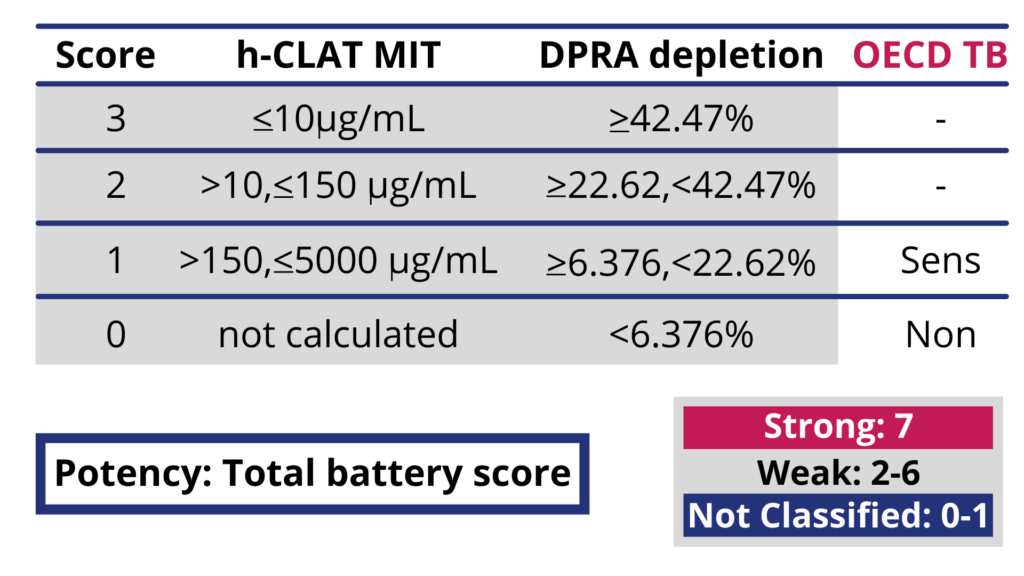
Also included in the guideline is the Kao Brands-developed Integrated Testing Strategy (ITS) Versions 1 and 2, incorporating in silico analysis of molecular structure in combination with a 2-assay in vitro evaluation of test materials. This approach provides the ability to resolve strong, weak (GHS Category 1A and 1B) and non-sensitizers. (see Figure 2).