Fig. 1a: The US EPA battery of acute toxicity tests. The blue dotted lines in association with the blue framed boxes indicate existing in vitro test methods adopted as OECD Test Guidelines and that could be considered for evaluation of their capacity.
In the current global regulatory climate favorable for further advancement of in vitro test methods, the US Environmental Protection Agency (US EPA) is driving significant efforts in the United States. The latest endeavor targets the modernization of the battery of acute toxicity tests classically known as the “six-pack” (Fig. 1a).
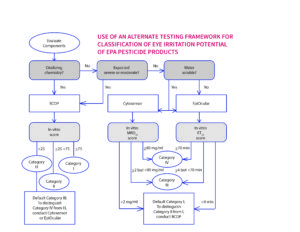
Known for many years as an “all in vivo” testing strategy for products registered under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), the six-pack has recently been revised to include non-animal test methods for classification and labeling for eye irritation. The policy is the outcome of a multi-year project between the US EPA, Institute for In Vitro Sciences, Inc. (IIVS), industry stakeholders, and coordinated by The Accord Group, to utilize non-animal (in vitro/ex vivo) test methods in place of the rabbit test to determine the eye irritation potential of commonly used household cleaning products with anti-microbial claims (Fig. 1b). In March of this year the US EPA updated this policy for the use of an alternate testing framework for classification of eye irritation potential of more conventional EPA pesticides products 1.
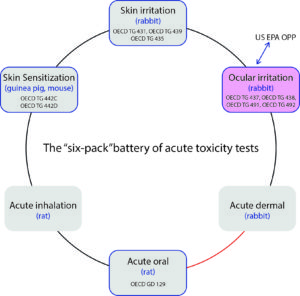
Fig. 1b: Decision tree: selection and evaluation of in vitro assays for hazard labeling when using an alternate testing framework for classification of eye irritation potential of US EPA pesticide products. Abbreviations: BCOP = Bovine Corneal Opacity.
The current efforts of the US EPA are also oriented to finalize the Process for Establishing & Implementing Alternative Approaches to Traditional In Vivo Acute Toxicity Studies, which was open to public comments early in 2015. To help with the reinvention of the six-pack, several validated in vitro test methods (many of which are adopted OECD Test Guidelines) could be considered for evaluation of their capacity to address US EPA hazard categories (Fig. 1a). Since most of these test methods have been validated to address hazard categories under the Globally Harmonized System (GHS), they could provide the needed framework to facilitate a potential alignment of US EPA to the GHS categorization systems.
IIVS has considerable knowledge and hands-on expertise using these in vitro test methods and can assist with US EPA regulatory submission needs. For more information, please contact us.
1 US EPA. Use of an alternate testing framework for classification of eye irritation potential of EPA pesticides products (2015).
